We support our Publishers and Content Creators. You can view this story on their website by CLICKING HERE.
Moderna has paused its mRNA RSV (Respiratory Syncytial Virus) vaccine trial for children after five infants were hospitalized with severe respiratory complications.
This comes just days after the FDA quietly admitted it had halted enrollment in similar trials, citing safety concerns for children under two years old.
The news broke during Thursday’s FDA Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting, where panelists struggled to navigate the safety implications of Moderna’s findings, Fierce Pharma reported.
Henry Bernstein, a professor of pediatrics at Hofstra/Northwell, stated, “This all seems like an incredible conundrum with lots of unanswered questions remaining—lots to still learn.”
The halted trial, identified as mRNA-1365-P101, sought to evaluate the safety, tolerability, and immunogenicity of two mRNA-based vaccines, mRNA-1345 and mRNA-1365. The vaccines were designed to protect infants and toddlers against RSV.
“FDA was notified of a study pause in Phase 1 study mRNA-1365-P101 due to a study pause criterion being met. A potential safety signal for RSV sLRTI was identified, and as additional information accrued, an imbalance in cases of RSV sLRTI was noted, with more cases identified in the vaccine groups compared with the control group. This raised a concern for possible VAERD,” according to the briefing document.
During the study’s younger cohort phase (5 months to <8 months>
- Five cases of severe RSV-LRTI occurred among vaccinated infants receiving lower doses of the vaccines.
- Only one case was reported in the placebo group, highlighting a concerning imbalance.
- Symptomatic RSV infections progressed to severe illness in 26.3% of vaccinated infants, compared to just 8.3% in the placebo group.
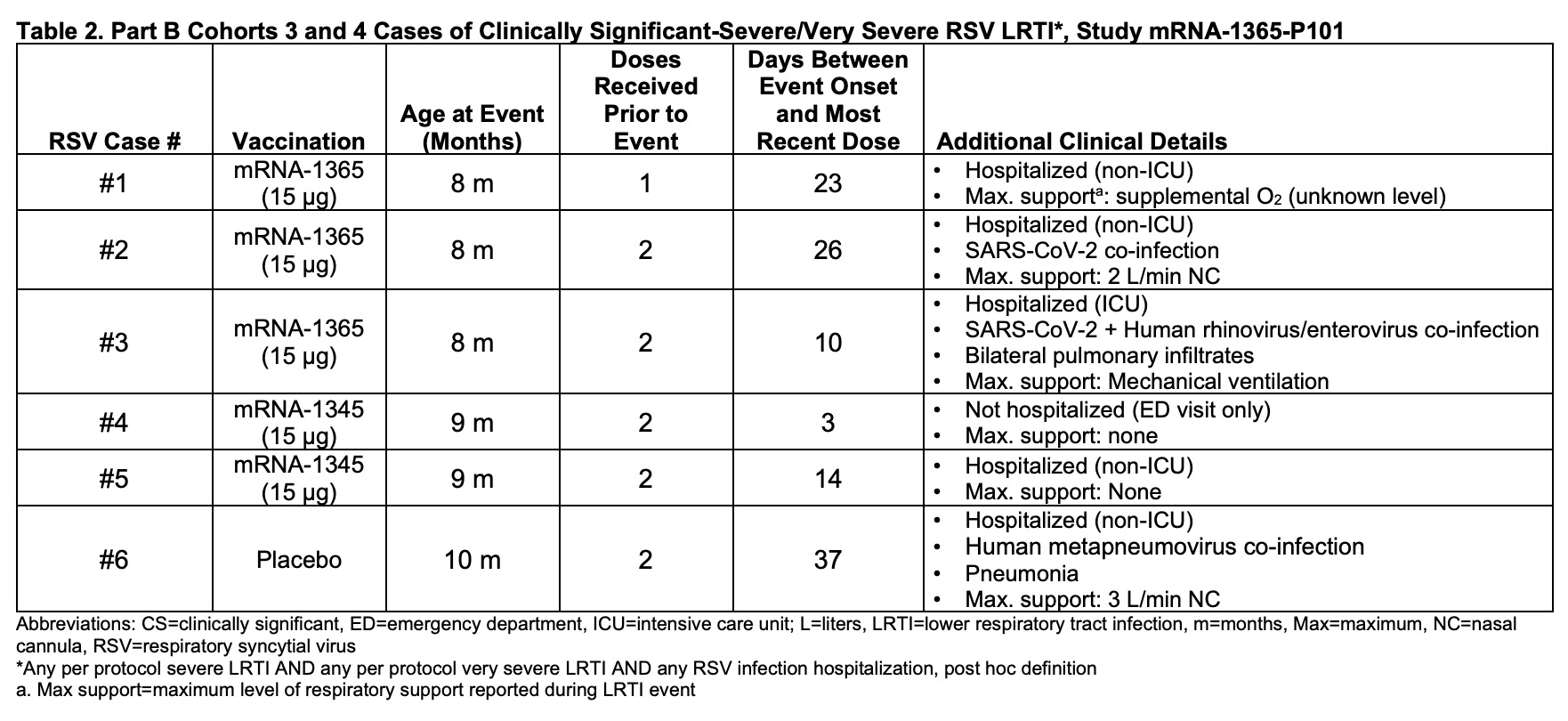
The trial’s pause was triggered by a pre-specified safety criterion: any severe LRTI with RSV-positive PCR in at least two participants. Once the threshold was reached, Moderna ceased enrollment and dosing across all study cohorts and immediately notified regulators, including the FDA.
The FDA placed the trial on a clinical hold, citing concerns over an “unreasonable and significant risk of illness or injury.” This pause affects the ongoing studies in infants aged 5 months to <24 months across multiple international sites>
While Moderna and the FDA claim to be exercising caution, critics argue that the real issue lies with the “vaccine-first” approach being pushed on an increasingly skeptical public.
According to Fierce Pharma, Dr. Arnold Monto of the University of Michigan admitted that the safety concerns must be treated seriously, calling for rigorous scrutiny of RSV vaccines “on a platform-by-platform basis.”
Yet, his comments highlight the elephant in the room: mRNA vaccines appear to carry unique risks that are not fully understood.

 Conservative
Conservative  Search
Search Trending
Trending Current News
Current News 





