We support our Publishers and Content Creators. You can view this story on their website by CLICKING HERE.
Using data from the Department of Defense (DOD) Joint Trauma System (JTS), The Remdesivir Papers came to life on October 4, 2024. In the 5,400-word report by J.M. Phelps and whistleblower Daniel LeMay (a pseudonym), a question was posed: “Are U.S. military treatment facilities and others hiding, or manipulating, the deadly results of clinical trials and more [emphasis added] surrounding the controversial drug purported to be a treatment for COVID-19?”
While the report focused largely on NCT04302766, it’s time to address the “and more.” Previously referred to as a clinical trial, NCT04302766 is correctly identified as an “expanded access treatment protocol” or “Protocol S-20-01 (IND 147993).
On September 27, Rep. Clay Higgins (R-LA) sent a letter to Defense Secretary Lloyd Austin, copying the U.S. Health and Human Services Secretary, the U.S. Food and Drug Administration Commissioner, U.S. Army Medical Research and Development Command (USAMRDC) Commanding General Major General Paula C. Lodi, and others. Rep. Higgins specifically requested information regarding NCT04302766, or the “Intermediate-Size Patient Population Expanded Access Treatment Protocol for Coronavirus Disease 2019 (COVID-19) Remdesivir (RDV; GS-5734™).”
In response to Rep. Higgins’ letter to Defense Secretary Lloyd Austin and others, a response was provided by the Office of the Under Secretary of Defense for Personnel and Readiness, also known as USD (P&R). The response verified that “under this expanded access protocol, patients received an initial 200 milligram (mg) intravenous (IV) dose of Remdesivir on day 1, followed by once-daily 100 mg IV dose on day 2 and up to day 10.” This falls in line with the standard dosage used by trials sponsored by the National Institute of Allergy and Infectious Diseases (NIAID). These include NCT04280705, NCT04401579, NCT04492475, and NCT04640168.
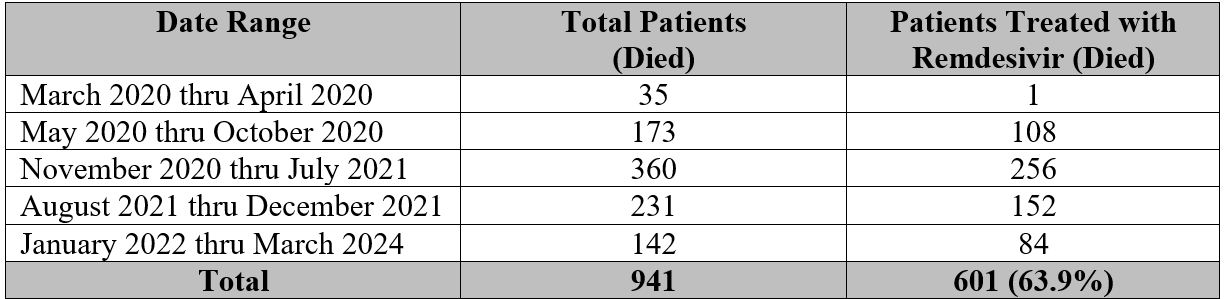
USD (P&R) also shared that “the [expanded access treatment] protocol enrolled a total of 40 patients between March 26, 2020 to September 10, 2020.” According to USD (P&R), only one patient died. Interestingly, this does not account for the 600 other service members and veterans noted with The Remdesivir Papers who were treated with remdesivir and died. Clearly, hundreds were subjected to the use of remdesivir outside the realm of the expanded access treatment protocol and this is the information now being sought by the author.
For the one patient participating in the expanded access treatment protocol who passed away, USD (P&R) said, “This patient experienced hypoxic respiratory failure, sepsis, leukocytosis (elevated white blood cell count), and anemia. There was no reasonable causal relationship between Remdesivir and this serious adverse event; it is believed the cause of death was likely due to complication from COVID-19.”
As previously noted in The Remdesivir Papers, anemia and septic shock are, in fact, potential organ-based adverse effects that could be attributed to the use of remdesivir. Apart from these side effects, what USD (P&R) failed to mention in the reply to Rep. Higgins is that the hypoxic respiratory failure can be preceded by acute respiratory failure—which is also a potential adverse effect of remdesivir.
Speaking to The Gateway Pundit, Dr. (D.O.) Samuel Sigoloff, an Army veteran and family medicine doctor, verified, “Remdesivir has the known adverse drug event complication of anemia which can happen in as many as 3.6 percent of patients.”
“Anemia does alter how much oxygen can carried through the blood which can lead hypoxic respiratory failure which is a type of acute respiratory failure,” Dr. Sigoloff explained. “Risk of death can be as high as 20 percent when one organ system fails, and the risk of death increases approximately 20 percent with each organ system that fails.”
While noting an elevated white blood cell count in the deceased patient, USD (P&R) also failed to mention that leukocytosis can be “a well-known predictor of chronic kidney disease (CKD) progression” and predictor of kidney function decline.
Considering the facts presented, to tell the congressman that “there was no reasonable causal relationship between Remdesivir and this serious adverse event” is, at best, very misleading.
Perhaps, it is an attempt to blame COVID-19, while masking the fact that—despite the drug’s long history of lethality—the Department of Defense and Department of Veterans Affairs had an obligation of nearly $12 million between 2019 and 2023 to Gilead Sciences, as well as authorized Veklury (remdesivir) distributors ASD Specialty Healthcare and AmerisourceBergen.
According to Dr. (Ph.D.) Crisanna Shackelford, a Navy veteran and leading advocate for service members and veterans affected by vaccine-related injuries, “It is both clinically and ethically indefensible to claim, without comprehensive and compelling evidence, that there is no reasonable causal relationship between Remdesivir and the serious adverse events leading to this patient’s death.” She told The Gateway Pundit that “given the known side effects and complications associated with remdesivir, including hypoxic respiratory failure, leukocytosis, sepsis, and anemia, any assertion of no causal link—without fully investigating these issues—fails to meet the standard of care or professional medical and legal due diligence.”
The Department of Defense must account for the other 600 out of 941 service members and veterans who were also treated with remdesivir and died. Are their spouses and families really expected to believe they all died from COVID-19?

 Conservative
Conservative  Search
Search Trending
Trending Current News
Current News 





